ABOVE: New research unveiled surprising connections between the brain and the heart. ©istock, NatalyaBurova
For decades, researchers have appreciated the intimate association between mental health and physical health, and studies suggest that the mind may impact various bodily systems.1 For example, high levels of stress rendered people more vulnerable to infections; conversely, mental health treatment reduced the risk of rehospitalization by 75 percent in people hospitalized for heart disease.2,3
However, the mechanisms by which mental states might influence the immune or cardiovascular systems are still not well understood. Asya Rolls, a neuroscientist at the Technion – Israel Institute of Technology, said that these questions are often overlooked because many researchers feel that the field of mind-body connection is not amenable to rigorous scientific exploration. “It’s a major, fundamental gap in our understanding of physiology and medicine, and our ability to help patients,” she said.

Previously, Rolls has used mouse models to demonstrate how brain regions associated with reward or emotion can modulate immune activity and inflammation in various contexts, including infection, cancer, and colitis.4–6 As evidence of the association between mental health and cardiovascular disease in humans accumulated, she wondered if she might be able to mechanistically study this heart-brain connection in mice.7 Together with cardiovascular medicine specialist Lior Gepstein and Hevda Haykin, a jointly supervised graduate student, Rolls set out to explore how particular neural systems influenced recovery from acute myocardial infarction (AMI) in a mouse model.
In a recent study published in Nature Cardiovascular Research, the researchers found that chemogenetically stimulating dopaminergic neurons in the ventral tegmental area (VTA), part of the brain’s canonical reward circuitry, improved left ventricular ejection fraction (LVEF; a measurement of the heart’s ability to pump out blood), increased vascularization, and decreased fibrosis in the 15 days following AMI.8 This mouse model not only allowed researchers to objectively measure the effects of brain activity on heart function, but also to explore the mechanisms underlying these effects.
“We know that when we experience stress or happiness—things that we might think of as moods—it does have an impact on peripheral organs and peripheral immune cells,” said Isaac Chiu, a neuroimmunologist at Harvard Medical School who was not involved in the study. “So, I thought it was fascinating that they made a connection between the brain’s reward system and heart attacks.”
After two weeks of stimulating the VTA in the treatment group, said Rolls, “It was pretty interesting to see the changes between the groups. But the real challenge was to figure out how [this was happening].”
In previous research, she demonstrated that VTA activation altered immune responses, so they first examined immune cells present in the heart. They found reduced numbers of cells positive for CD68, a marker found on monocytes and macrophages. The significance of this finding is not entirely clear, however: CD68+ cells are diverse, and play complex and incompletely understood roles in both healing and detrimental changes to the structure of the heart after AMI.9
Furthermore, Rolls said that the research team was skeptical that the observed immunological changes alone could account for the considerable differences they detected in heart physiology. To determine additional factors that might contribute to these disparities, they analyzed the proteomic profiles of the hearts.
“Once we did proteomics, it became pretty clear that most of the changes that we were seeing—in addition to all these immune changes—were changes in proteins that were secreted by the liver,” said Rolls. “Originally, I would have never bet on the liver! But that’s the fun of science.”
The proteomic analysis revealed several upregulated proteins, but the team homed in on one for further exploration: complement component 3, or C3. This protein is best known for its role in innate immunity but may also play an important role in tissue regeneration.10 While VTA stimulation did not increase C3 mRNA in the heart, it did increase C3 mRNA in the liver, suggesting that this protein is manufactured in the liver in this particular context. Supporting the protein’s role in cardiac recovery, researchers found that administering a C3 inhibitor blocked the improvements in heart function observed following VTA stimulation.
Rolls noted that while this indicates that C3 is essential for these processes, it doesn’t mean that it is the only important protein. “There are many other factors that were affected in our screen,” she said. “And these may be other components that we never thought of as relevant targets for treating [myocardial infarction], but they actually might be.”
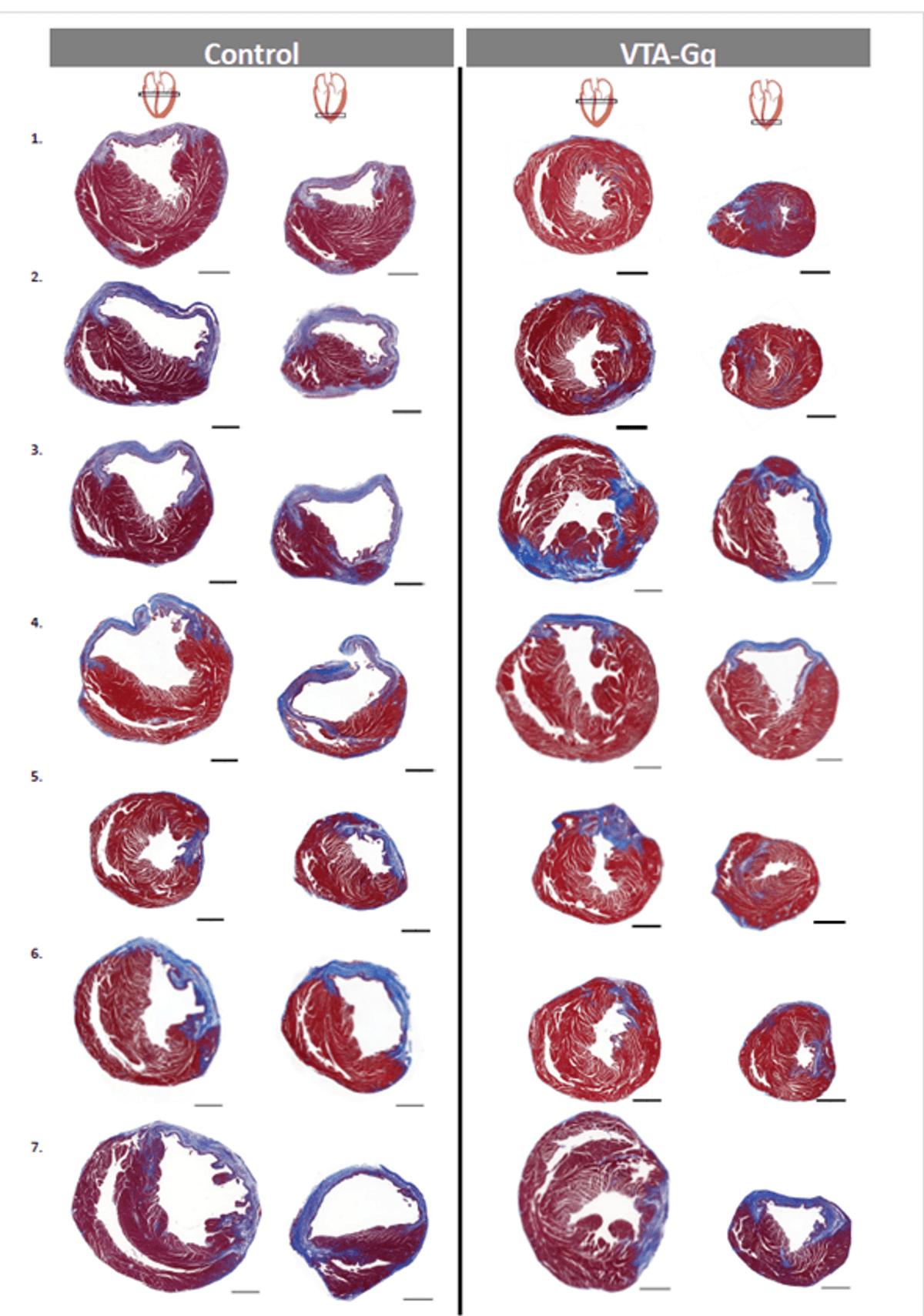
While there were no significant differences in survival over the 15 days following AMI, the researchers hypothesized that longer-term studies might reveal differences, as the untreated mice had substantially worse heart function and decreased LVEF. Previous studies in humans have indicated that decreased LVEF is associated with increased mortality in the months or years following AMI.11
Improvements in the scientific understanding of the ways in which the brain can influence peripheral systems could also inform new therapeutic possibilities. In the future, certain cardiovascular or immune conditions could perhaps be treated with non-invasive neuromodulation techniques, such as neurofeedback, transcranial magnetic stimulation, or focused ultrasound, said Rolls.
This work could also help researchers understand the mechanisms that link psychological factors with physical health, such as the association of optimism with lower rates of cardiovascular disease and all-cause mortality.12
However, Rolls cautions that this research is still in the early stages of development. “All our work at this point is really, really basic,” she said. “I'm always terrified that patients at some point will think, ‘okay, I don't need the drugs, I can cure myself with positive thinking.’ That's my biggest fear.” Instead, targeting psychological factors could be just one tool that physicians use to prevent or treat disease.
“I think [this study] is very important,” said Chiu. “There are many things that can activate the reward system or motivation system, and this study implies that does have an impact on the outcome of heart attacks. That could be quite clinically relevant.”
For her part, Rolls will continue to explore the language of mind-body interactions. “We're really interested in how the brain represents different physiological states, and then understanding which pathways it uses to solve these conditions.”
Disclosure of Conflicts of Interest: Study authors Asya Rolls, Lior Gepstein, Hevda Haykin, and Hilla Azulay-Debby have filed a patent that includes the proteomics analysis covered in the study.
- Vaillant GE. Natural history of male psychologic health: Effects of mental health on physical health. N Engl J Med. 1979;301(23):1249-1254.
- O’Connor DB, et al. Stress and health: A review of psychobiological processes. Annu Rev Psychol. 2021;72:663-688.
- Carmin CN, et al. Impact of mental health treatment on outcomes in patients with heart failure and ischemic heart disease. J Am Heart Assoc. 2024;13(7):e031117.
- Ben-Shaanan TL, et al. Activation of the reward system boosts innate and adaptive immunity. Nat Med. 2016;22(8):940-944.
- Ben-Shaanan TL, et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat Commun. 2018;9(1):2723.
- Koren T, et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184(24):5902-5915.e17.
- Kwapong YA, et al. Association of depression and poor mental health with cardiovascular disease and suboptimal cardiovascular health among young adults in the United States. J Am Heart Assoc. 2023;12(3):e028332.
- Haykin H, et al. Reward system activation improves recovery from acute myocardial infarction. Nat Cardiovasc Res. 2024;3(7):841-856.
- Peet C, et al. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116(6):1101-1112.
- Zhang C, et al. Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking. Nat Commun. 2017;8(1):2078.
- Møller JE, et al. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. 2006;151(2):419-425.
- Krittanawong C, et al. Association of optimism with cardiovascular events and all-cause mortality: Systematic review and meta-analysis. Am J Med. 2022;135(7):856-863.e2.




