Stay up to date on the latest science with Brush Up Summaries.
What Is Western Blotting?
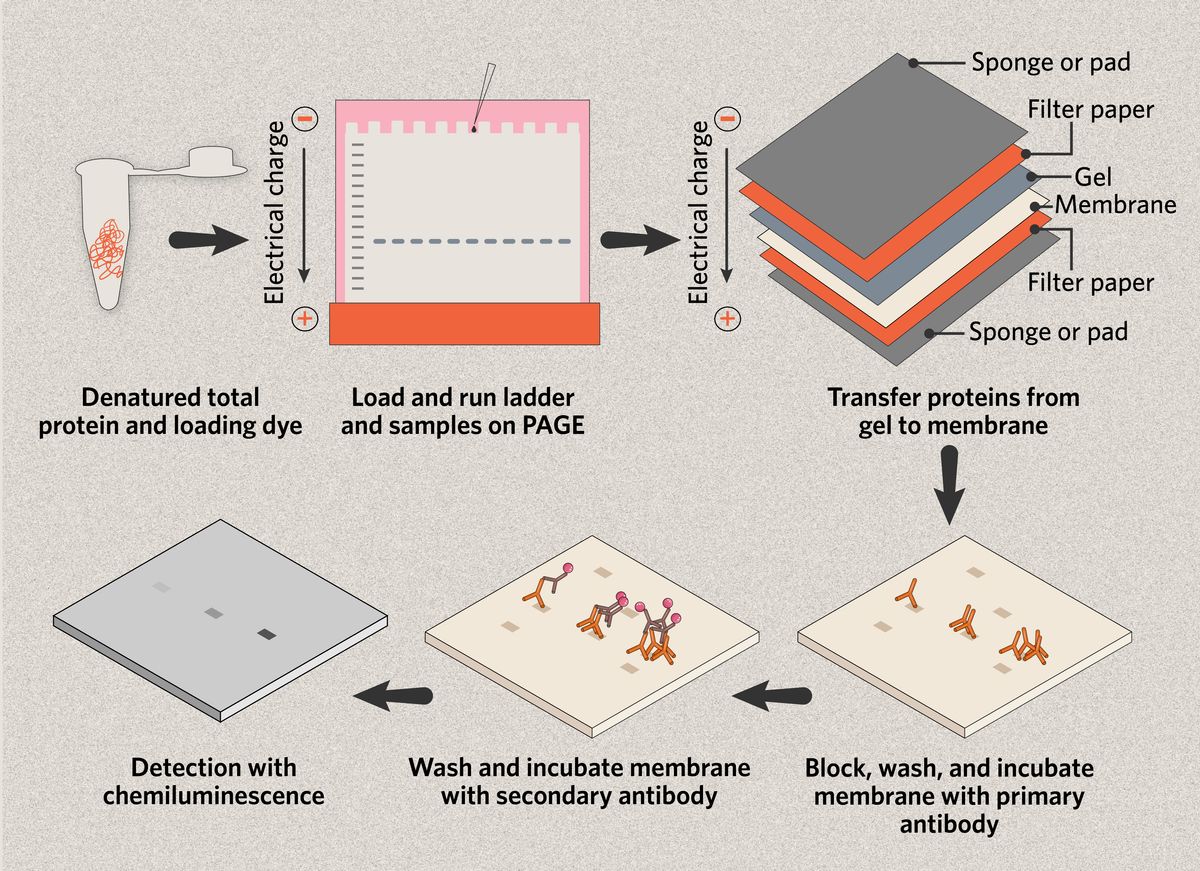
Western blotting, otherwise known as immunoblotting, is a common molecular biology technique that scientists use to detect and analyze proteins in a biological sample.1 Total proteins from the sample are separated by size via gel electrophoresis, then transferred onto a nitrocellulose membrane, where specific target proteins are labeled using antibodies so that they can be visualized.2
Southern, Northern, and Western Blot: What’s The Difference?The main difference between Southern, northern, and western blot techniques is the target molecules. While western blots target proteins in a sample, Southern blots reveal DNA and northern blots indicate RNA.3 These techniques typically use similar size separation and transfer methods, but different binding and detection approaches. |
What Are Western Blots Used For?
Western blots do not determine the absolute abundance of a target protein in a sample but rather the presence or absence of the target protein in a sample, as well as the relative abundance of the target protein between different samples.1
For example, if a scientist wants to produce large quantities of a particular protein in an immortalized cell line in vitro but is not sure which cell line to choose for this application, they could take several different common cell lines, such as HEK293, HeLa, and CHO cells, transfect a flask of each cell line with a target gene, and grow the cells for a few weeks before analyzing their protein content by western blot.
By extracting and measuring the total protein concentration from each cell line and performing a western blot, the researcher can determine which cell lines are able to express the protein, and of those that do produce the protein, which produces the most.
Western Blot Protocol
The main steps in a western blot protocol are as follows.
- Sample preparation, including protein extraction and quantification
- Protein separation via gel electrophoresis
- Membrane transfer of proteins from the gel
- Immunoblotting with primary and secondary antibodies
- Target detection
Western blot sample preparation: Protein extraction and quantification
Sample preparation is a crucial first step in performing an accurate western blot.4 After extracting total protein from the cells or tissue, researchers must measure the concentration of protein in each sample, for example using a Bradford assay.
Most western blot protocols suggest a maximum of 50µg of total protein per sample; an equal amount of total protein between samples is critical for any type of quantitative comparison.4 Loading too much total protein can cause problems such as the decreased binding of low-abundance proteins due to the abundant proteins saturating the membrane.2
Protein separation via gel electrophoresis

The next step in a western blot protocol is polyacrylamide gel electrophoresis (PAGE),3 and scientists can use one of two main approaches in this stage of sample preparation. The first is to run the native proteins through the gel, separating them by their charge, mass, and structure (native PAGE).4
The second method is to denature the sample by heat, unfolding the proteins and breaking up any protein complexes, and coating them in sodium dodecyl sulfate (SDS), an anionic detergent that gives all the proteins a negative charge. This approach allows the proteins to be separated only by their mass (SDS-PAGE).4
Researchers load the samples into the wells of a thin polyacrylamide gel, where an electrical current pushes the proteins through the gel matrix; because smaller proteins pass through the gel more quickly, they are separated by size.2 A protein ladder containing a range of known molecular weights is included in one well to determine the size in kilodaltons (kDa) of the target protein.
Membrane transfer
Once the proteins have been size-separated via PAGE, scientists transfer the proteins onto a membrane, typically made of nitrocellulose or polyvinylidene fluoride (PVDF).5 The gel is carefully placed over the membrane and sandwiched between filter papers and absorbent pads that are pre-soaked in buffer. An electrical current is then applied to migrate the proteins from the gel onto the membrane.3
At this stage, scientists often stain the membrane with a nonspecific reversible protein-binding dye such as Ponceau S or Coomassie blue to determine if the protein transfer has been successful and if there are any imperfections from air bubbles between the gel and membrane during transfer that will affect the resulting image.4
Immunoblotting with primary and secondary antibodies
After transfer, researchers incubate the membrane with antibodies to enable target detection. Blocking with milk proteins or bovine serum albumen (BSA) before and during immunoblotting helps prevent nonspecific antibody binding on the membrane.6 The primary antibody, specific to the target protein of interest, is diluted in buffer and incubated with the membrane. Incubation time and conditions vary between antigens and antibodies.
Multiple wash steps between the primary and secondary antibody incubations remove any unbound primary antibody and reduce background signal.6 The secondary antibody can then be applied. Designed to bind to the primary antibody, this secondary antibody is conjugated with an enzyme, radioactive isotope, or a fluorescent dye that will enable detection.3
Target detection
Scientists use imaging tools to visualize western blots and detect the target protein.7 Enhanced chemiluminescence (ECL) is the most common method for the detection of target proteins in western blotting,5 but fluorescence is also popular. Because the secondary antibody binds only to the target-specific primary antibody, the imaging process will produce a band on the membrane if the target is present in a sample.
Western Blot Analysis: How to Read a Western Blot
Bands on a western blot image reveal the presence, absence, and relative abundance of the target protein.2 If there is no band present for a sample, the target is absent or may be at undetectably low concentrations. If bands are present, the intensity of the band may reveal the relative quantities of the target between samples;3 a strong band may indicate there is more of the target protein present in a particular sample, although too strong of a signal may indicate over-saturation that is less informative for determining relative protein abundance.
Returning to the earlier example of a scientist trying to decide which cell line to use to produce a protein of interest, the researcher can compare expression between HEK293, HeLa, and CHO cells by imaging a western blot. If they see no band present for the HEK293 sample, a faint band for HeLa, and an intense band for CHO, these results indicate the following.
- HEK293 cells are unable to produce the protein of interest
- Both HeLa and CHO cells can produce the protein of interest
- CHO cells can produce the most target protein
Blotchy Western Blot Troubleshooting Tips
Western blots are notorious for producing poor-quality images and this can be due to a variety of factors. A common issue that contributes to a dark, blotchy western blot is air bubbles between the gel and the membrane during protein transfer. A small roller should be used to gently and thoroughly remove any air pockets or bubbles from the gel sandwich, ensuring complete contact between the gel and the membrane.
Letting the membrane dry out at any stage can also cause the resulting image to be dark and splotchy, making it difficult to detect bands. Gentle agitation should be applied during incubations to ensure even and continuous exposure of the membrane to the solution.
Nonspecific bands can also be present on a western blot, often due to insufficient blocking, which can contribute to high background signal.2 Additionally, primary antibodies must be high quality, validated with positive and negative controls, and used at the optimal concentration for the target protein to avoid nonspecific binding.2
Western Blot Applications
The western blot assay has a multitude of applications, from basic to applied research. Scientists often use western blots as a diagnostic tool. For example, researchers developed an HSV western blot test to determine the presence or absence of antibodies against herpes simplex virus-2 (HSV-2) in dried blood spot samples.8
Similarly, scientists have used a western blot test in the diagnosis of Lyme disease since the early 1990s.9 Researchers also use western blots for biomarker measurements in the drug development process.10
- Taylor SC, et al. A critical path to producing high quality, reproducible data from quantitative western blot experiments. Sci Rep. 2022;12(1):17599.
- Ghosh R, et al. The necessity of and strategies for improving confidence in the accuracy of western blots.Expert Rev Proteomics. 2014;11(5):549-560.
- Alberts B, et al. Molecular Biology of the Cell. 5th ed. (Anderson M, Granum S, eds.). Garland Science; 2008.
- Sule R, et al. Western blotting (immunoblotting): History, theory, uses, protocol and problems.Biotechniques. 2023;75(3):99-114.
- MacPhee DJ. Methodological considerations for improving Western blot analysis. J Pharmacol Toxicol Methods. 2010;61(2):171-177.
- Begum H, et al. Western blotting: A powerful staple in scientific and biomedical research. Biotechniques. 2022;73(1):58-69.
- Gingrich JC, et al. Multiplex detection and quantitation of proteins on Western blots using fluorescent probes. Biotechniques. 2000;29(3):636-642.
- García-Cisneros S, et al. Performance of ELISA and Western blot to detect antibodies against HSV-2 using dried blood spots. J Infect Public Health. 2019;12(2):224-228.
- Dressler F, et al. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167(2):392-400.
- Owen C, et al. Western blotting: Evolution of an old analytical method to a new quantitative tool for biomarker measurements. Bioanalysis. 2024;16(5):319-328.

