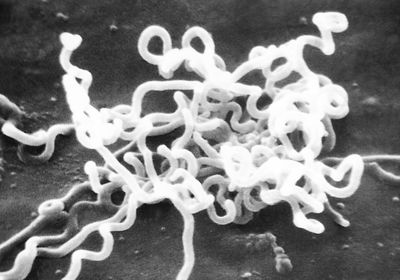
ABOVE: A 1980 scanning electron microscopic image of Treponema pallidum bacteria, the causative agent of syphilis CDC/DAVID COX
Syphilis has long been difficult to eradicate—and it’s having another resurgence. In the United States alone, more than 171,000 cases of the sexually-transmitted infection were reported in 2021, up 68 percent since 2017, according to preliminary data from the Centers for Disease Control and Prevention. Especially concerning, doctors say, are cases of congenital syphilis, where the infection is passed from a pregnant person to their fetus during pregnancy, which have nearly tripled in the same time period.
The trend isn’t entirely new. After a sharp decrease in syphilis cases in the 1940s and 1950s in the United States and the United Kingdom, credited to the availability of penicillin and widespread screening, there have been cycles of rises and declines. Over the course of the 1990s, cases dropped, but they began to rise again in 2000. Although the exact cause of the current resurgence is not well understood, Caroline Cameron, a professor of biochemistry and microbiology at the University of Victoria in Canada, suggested it may be partly due to an increase in condomless sex linked to new HIV/AIDS prophylactics, illicit drug-use, and the proliferation of dating apps.
Despite being one of the oldest known sexually transmitted infections, with possible origins in the 14th century—as well as intense public health initiatives over the past many decades—syphilis receives little attention from researchers. In fact, “only a handful of labs in the world are currently working on this important disease and its causative agent," Sheila Lukehart, a professor emeritus of medicine and global health at the University of Washington, wrote in an email to Undark.
This is in part because the bacterium that causes syphilis, Treponema pallidum, “is fragile to work with,” said Cameron. “So, you can't use regular experimental methods to work with it.”
Because of that fragility, researchers have been limited in their ability to develop new syphilis diagnostics, treatments, and preventive measures such as vaccines. Effective treatments are additionally challenging, experts say, because of T. pallidum’s ability to evolve resistance to antibiotics. Left untreated, in about 15 to 30 percent of infected people, the disease can permanently damage the brain, heart, and other organs and be life-threatening. Congenital cases can cause birth defects, stillbirth, and premature death.
New techniques to grow the bacteria in the lab may make it easier to study syphilis. But doing so will require more researchers focused on the disease. “There is an entire generation of clinicians and researchers who may have never seen or thought about syphilis,” said Ina Park, an associate professor of family community medicine at the University of California, San Francisco. Now, she added, “we need to catch up.”
The main limitation for syphilis research has been an inability to grow the delicate bacteria that causes it in the lab. Humans are the only natural host, although in research studies, scientists have been able to infect other animals with T. pallidum.
There were many false starts to growing T. pallidum outside of human and animal bodies, but it wasn’t until the 1970s that researchers found that the bacteria needed both mammalian cells and low oxygen levels for a chance to survive. There was little success, however, in getting the bacteria to thrive in these conditions long enough for an experiment. This changed in 1981, when researchers in California reported a successful method in which T. pallidum could be cultivated and multiplied in the skin cells of cottontail rabbits, mixed with key nutrients.
Still, the process wasn’t easy, and for many years, experiments on rabbits—animals exhibiting infections similar to humans—were the only option for research studies, an expensive endeavor that requires large animal facilities.
But in 2018, scientists at the University of Texas Health Science Center in Houston developed a new technique for long-term cultivation of T. pallidum. In this method, researchers once again used cottontail rabbit skin cells, but they improved the conditions by adding even more nutrients to the mix. Researchers found that such bacteria could grow for more than three years and retain their structure and ability to move, multiply, and cause infection—meaning that experiments using these bacteria could be informative for understanding syphilis.
In 2021, a team of scientists used this technique for a study in which they identified an antibiotic known as linezolid as a possible syphilis treatment. This discovery is especially important, research suggests, because some people are allergic to penicillin, which has traditionally been the first line of defense against syphilis. Penicillin is also often in short supply in many parts of the world. The effectiveness of linezolid against syphilis is set to be tested in humans in a clinical trial.
While anyone who is sexually active can get syphilis, men who have sex with men are at higher risk. A study published in The Lancet Global Health estimated that prevalence of syphilis is 15 times more in this demographic compared to men in general population. The resurgence in syphilis is primarily attributed to increased cases in this high-risk population.
The reasons for increased syphilis prevalence among men who have sex with men are complex. Some researchers point to a decrease in condom use, in part because of improved HIV following antiretroviral treatments, although the evidence supporting this claim is inconsistent. Other research suggests that an increase in sexual partners due to dating apps, as well as sexualized drug use, could also play a part.
A 2019 clinical trial treated men who have sex with men and were either HIV-positive or taking medication to prevent infection in San Francisco and Seattle with the antibiotic doxycycline. According to preliminary results of the study, the treatment—known as doxycycline post-exposure prophylaxis—was effective in preventing syphilis when taken within three days of condomless sex.
San Francisco’s public health department rolled out doxycycline post-exposure prophylaxis, or doxycycline PEP, in October 2022 to help combat sexually transmitted diseases, especially syphilis.
But the move has not been without controversy. Researchers have worried, for instance, about potential antibiotic resistance that may limit treatment options for other conditions addressed with doxycycline, such as skin infections and bacterial pneumonia.
Lukehart told Undark that T. pallidum has a remarkable ability to develop antibiotic resistance and that it would be “impossible to keep physicians from prescribing” doxycycline more broadly, an approach that “is almost certainly likely to be widely used, particularly in countries where antibiotics are readily available without prescription.”
Still, some researchers see potential in doxycycline-PEP. “I believe it could be very effective, especially if used by the people who are most at risk for syphilis,” said Park, who also noted that the drug is cheap, widely available, and has been safely used for decades with few side-effects.
“However, if there are unintended negative consequences,” Park said, “we may not be able to reverse course.”
Researchers will also need to improve diagnostic tests for syphilis. Those currently in use “can’t differentiate active versus previous infections, because an individual’s antibodies against T. pallidum that they generate will remain present for their lifetime,” Cameron said.
Success in treating syphilis is normally assessed according to the decline in levels of specific antibodies. But in about a quarter of people who have been infected with syphilis, the antibody levels don’t drop after treatment. In these cases, even repeated testing cannot distinguish between new and old infections, which means doctors don’t have enough information for treatment.
A solution would be a test that directly detects syphilis-causing bacteria through its DNA or proteins. In February 2022, Cameron received a $2 million grant from Open Philanthropy, a San Francisco-based funder, to help develop such a test—one that looks for proteins in patient samples including urine and plasma, the clear liquid portion of blood.
Beyond improved testing and treatments, there is wide agreement among the experts who spoke with Undark that a vaccine would be the best solution. “As syphilis is only transmitted among humans, an effective vaccine has the potential to eliminate the disease altogether, thus also preventing the development of antibiotic resistance in this bacterial pathogen,” Raymond Tsang, a research scientist and laboratory chief at the Public Health Agency of Canada, wrote in an email to Undark.
The progress on a vaccine has been slow. So far, just one study reported complete protection against T. pallidum infection—in rabbits. That study was done 50 years ago and required 60 doses of inactivated, whole T. pallidum bacteria.
Since then, experts including Cameron and Lukehart have identified some of the bacterium’s proteins that are responsible for spreading the disease within the body and between people. Lukehart and colleagues recently published a study on rabbits to test a potential vaccine that targets these proteins. Although the vaccine failed to provide complete protection against infection, it did reduce transmission or spread.
Christopher Kenyon, a professor at the Institute of Tropical Medicine in Antwerp, Belgium told Undark that although the results could be stronger in the rabbit study, the research is “very exciting.”
He added: “I think every small bit helps.”
Bhargavi Duvvuri is a fellow in global journalism at the Dalla Lana School of Public Health at the University of Toronto.
This article was originally published on Undark. Read the original article.