Article reviewed by Grant McFadden, PhD from Arizona State University and Kalivir Immunotherapeutics.
Stay up to date on the latest science with Brush Up Summaries.
What Are Oncolytic Viruses?
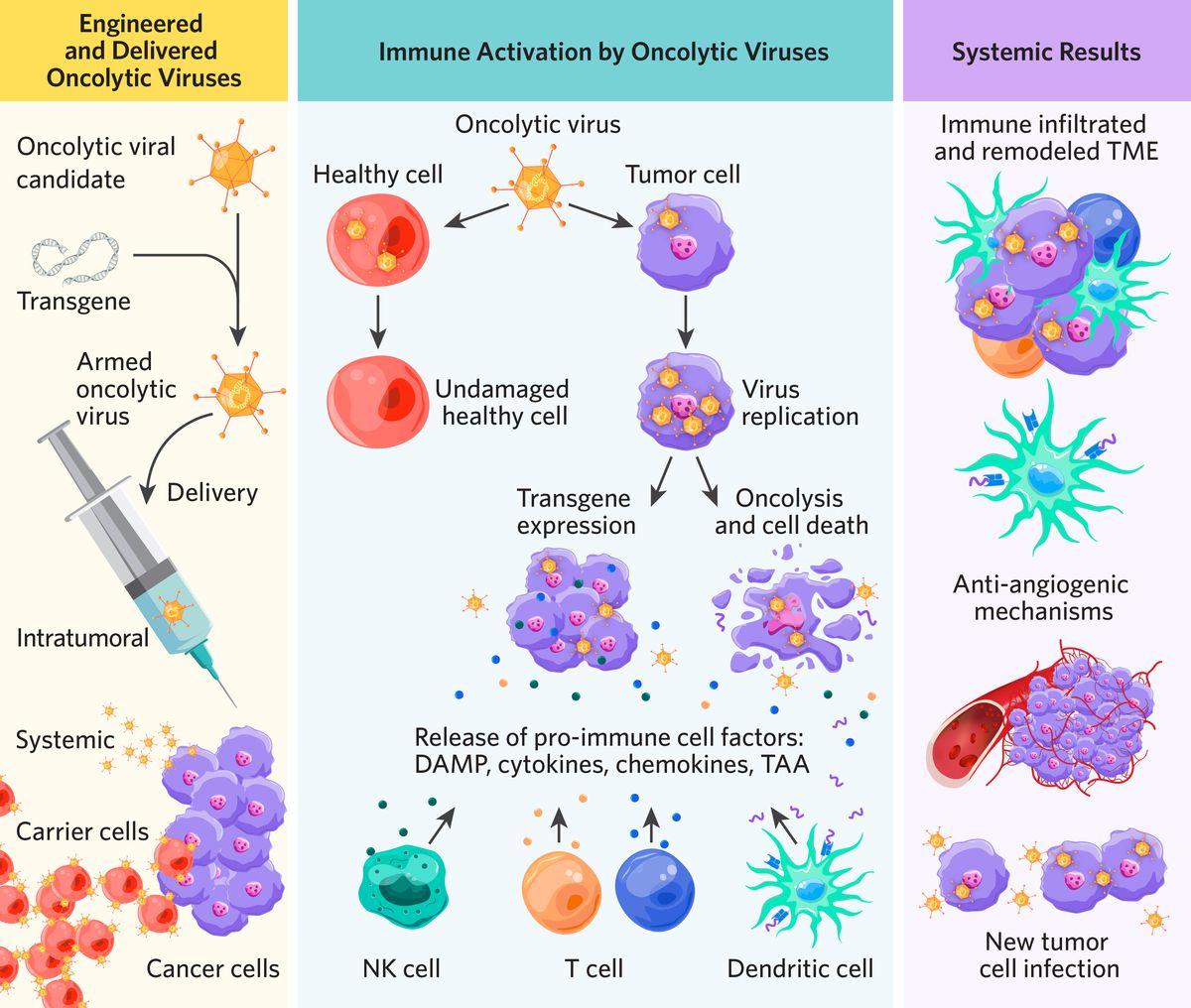
Scientists genetically engineer oncolytic viruses to selectively infect and replicate inside tumor cells, curbing cancer growth.1 Through genetic engineering, oncolytic viruses become sophisticated biological tools capable of complex treatment protocols tailored for heterogeneous cancers.2 These modified viruses hold significant promise, especially when integrated with complementary therapies.2
Oncolytic virotherapy stands out as a promising immuno-based anticancer strategy currently undergoing extensive evaluation in clinical and experimental trials.3 By incorporating specially selected genetic material called transgenes into viruses, scientists engineer oncolytic viral therapies to enhance current cancer treatments and the body’s natural antitumor response.2 Transgenes can serve several purposes when expressed in cancer cells, including the following.1-3
- Inducing cancer cell apoptosis
- Igniting an immune response within tumors
- Recruiting antigen-presenting cells
- Amplifying antitumor T cell responses
- Promoting the transcription of therapeutic agents
Types of Oncolytic Viruses
There are a wide range of oncolytic viral candidates, including both DNA and RNA viruses.2 Scientists choose between viral candidates based on their application needs. “One of the benefits to the larger DNA viruses is they can accept multiple transgenes,” said Grant McFadden, a retired virologist at Arizona State University and Board of Directors member of Kalivir Immunotherapeutics, which develops virotherapies to treat solid tumors.
DNA viruses such as adenoviruses can express high-fidelity enzymes but may pose genome integration risks.3 Conversely, RNA viruses offer efficient delivery and are effective against central nervous system tumors, with the ability to cross the blood-brain barrier, but lack high quality enzymes.3
Viral vectors in clinical trials include the following.1,2,4
- Adenoviruses
- HSV (including HSV-1 and HSV-2)
- Reovirus
- Vaccinia virus
- Myxoma
- Measles
- Coxsackie virus
Ongoing clinical trials continue to demonstrate the safety and efficacy of oncolytic virotherapy across a range of cancer types.1 In 2015, the US Food and Drug Administration (FDA) authorized talimogene laherparepvec (T-VEC), the first intratumoral HSV-based treatment for metastatic melanoma.2
How Do Oncolytic Viruses Infect Tumor Cells?
By harnessing a vector virus’s natural tropism, researchers create oncolytic viruses that navigate to tumor sites and selectively attack cancer cells.1
Physical delivery methods for oncolytic virotherapy
The most common delivery method is intratumoral injection, which is ideal for superficial lesions such as melanoma.1 This method avoids systemic side effects but may pose risks like deep tissue injury and limited immune response activation in distant tumors.3
Intravenous delivery offers accessibility but faces challenges such as hemodilution, sequestration in non-target organs, and rapid viral clearance by the immune system.1 Pairing novel techniques such as magnetic guidance, hydrogels, and ultrasound with non-intratumoral delivery aims to refine delivery precision and reduce toxicity.1
Researchers are pioneering innovative strategies to transport or shield oncolytic viruses from the immune system during delivery, increasing infection levels. These include the following.1,2
- Stem cells including mesenchymal stem cells (MSCs)
- Chimeric antigen receptor (CAR)-engineered T cell therapy
- Natural killer cells
- Bacteria
- Nanomaterial coatings
- Biomineral shells
Ongoing research develops strategic delivery methods to aid viral navigation around biological barriers in and around the tumor microenvironment (TME).1 Solid tumors often have additional obstacles such as increased fluid pressure, dense extracellular matrixes, and immunosuppressive conditions that limit viral invasion and immune cell infiltration.1,5
Oncolytic virus selectivity for cancer cells
Through genetic engineering, scientists enhance the ability of oncolytic viruses to selectively target cancer cells.1 One method for increased cancer cell selectivity is imbuing oncolytic viruses with a new type of transgene called bispecific T cell engagers (BiTEs).6 When cancer cells express BiTEs on their surface, the BiTEs bind to T cells, encouraging reactive T cell cytotoxicity.
While some oncolytic viruses target specific cell surface receptors, others differentiate between healthy and tumor cells after infection. “Some viruses are very promiscuous,” explained McFadden. “They will infect any cell they can get their paws on, but they distinguish a normal or a cancerous cell once they get inside.” This approach leverages gene expression disparities between healthy and cancerous cells such as abnormal signaling pathways or the high expression of specific promoters. By placing viral transgenes under tumor-specific promoters, they are primarily expressed in tumor tissues while sparing healthy cells.3
How Do Oncolytic Viruses Cause Tumor Cell Death?
Apoptosis with oncolytic viruses
Some oncolytic virotherapies target and destroy tumor cells through a process called oncolysis, which induces cancer cell death through apoptosis.3 However, this is not always oncolytic virotherapy’s outcome “and there are other cell death pathways (such as necroptosis) that are thought to be even more stimulatory to the immune system” explains McFadden. “Most viruses are interested in simply making copies of themselves and whether or not they kill the cell that they infect depends upon their biology,” said McFadden. “Sometimes it’s just an accidental byproduct of what the virus does.” Oncolytic viral replication may also inhibit cell protein production, disrupt cellular function, and induce oxidative stress, which can cause cell death. 1,3Additionally, researchers may incorporate tumor suppressor genes such as TP53 and PTEN into oncolytic viruses to facilitate tumor apoptosis.1
Targeted therapy and arming with oncolytic viruses
Oncolytic viruses serve as vectors, inducing cancer cells to express therapeutic genes, enhancing tumor-specific targeting, and reducing off-target effects.3 This strategy, known as arming, combats the drug resistance associated with conventional single-target anticancer drugs.3
One emerging arming technique in oncolytic virotherapy uses transgenes to make cancer cells more sensitive to a variety of medications called prodrugs, which are inactive compounds that the body metabolizes into active therapeutics.7 When an oncolytic virus transfects cancer cells with drug sensitivity genes, the cells become susceptible to nontoxic or low-toxicity prodrugs.3
Alternatively, oncolytic viruses can arm cancer cells with prodrug-converting enzymes that transform inactive drug precursors into cytotoxic agents at the cancer site.7 The prodrug becomes cytotoxic at the tumor bed, reducing systemic toxicity and preserving healthy cells.
Oncolytic viruses alter the physical tumor microenvironment
Scientists can genetically modify oncolytic viruses to express enzymes that degrade extracellular matrices, improving the ability of viruses or other therapies to navigate complex tumor environments.3
Oncolytic viruses can encode anti-angiogenic transgenes that code for proteins such as interleukin-12, which inhibits new blood vessel formation within tumors and induces vascular collapse, ultimately leading to tumor cell death.8
How Do Oncolytic Viruses Alter the Immune System?
To be effective, oncolytic viruses must first evade viral clearance by the immune system, then infect cancer cells and cause cell death that stimulates endogenous immune cells to eliminate additional cancer cells.1,3
“Everyone wants a kind of cell death called ICD or immunogenic cell death,” McFadden said. “That simply means that when the cancer cell dies, it is highly pro-immunogenic. It is highly stimulatory to the acquired immune system.” When cancer cells experience ICD, a cascade of immune events follows.1,9
- Release of immune components such as tumor-associated antigens (TAAs), cytokines, and chemokines
- Activation of autophagic pathways
- Stimulation of CD4+ and CD8+ T cell
- Migration of additional immune cells to the tumor bed
Increased immunogenicity can lead to an enduring antitumor immune response and prevent tumor recurrence.1,2
Oncolytic virotherapy triggers tumor microenvironment changes with cytokine and chemokine release
Cold tumor microenvironments are immunosuppressive and devoid of immune cells. Oncolytic virotherapy has the unique ability to transform these immunosuppressive TMEs into immunogenic environments known as hot tumors.3 Next-generation oncolytic viruses aim to accomplish this by inducing immune infiltration, which drives robust and sustained antitumor immunity and cancer regression.
Scientists achieve this transformation from cold to hot by incorporating cytokine or chemokine encoding genes into viral genomes, which enhance therapeutic effects by boosting local immune response.1-3
Cytokines and chemokines can trigger the following.1,3
- Promote immune cell maturation
- Increase local immune inflammatory mediators
- Guide immune cells to TME
- Regulate antitumor response
Research in various tumor models suggests that incorporating cytokines such as TNF-alpha, GM-CSF or IL-12 into oncolytic viruses activates antitumor T cell responses and induces tumor eradication.1
Future Research and Clinical Applications for Oncolytic Virotherapy
Evidence from clinical trials suggests that technical advances in potency and delivery efficacy are needed for oncolytic virotherapy to become a potent weapon in the fight against cancer.1 Current obstacles include the following.1
- Biological barriers to tumor access
- Tumor heterogeneity
- Antibody neutralization in the blood
- Immunosuppressive TMEs
- Low viral payloads
“Making clinical grade virus is complex,” said McFadden. “Methods have to be derived for every single virus and they're quite unique for every virus. That’s an expensive proposition.”
As clinical trials continue to demonstration, strategically combining cancer therapies with oncolytic virotherapy enhances efficacy by promoting intratumor viral spread and immune activation.2 Complementary pairings include the following.1,10
These integrated approaches widen therapeutic windows, potentially reducing toxicities and shaping a promising frontier in cancer treatment.1
Grant McFadden is a Board of Directors member for Kalivir Immunotherpeutics.
- Ji Q, et al. Strategies for advanced oncolytic virotherapy: Current technology innovations and clinical approaches. Pharmaceutics. 2022;14(9).
- Russell SJ, et al. Advances in oncolytic virotherapy. Commun Med (Lond). 2022;2(1):33.
- Yang L, et al. Oncolytic virotherapy: From bench to bedside. Front Cell Dev Biol. 2021;9.
- Chan W, et al. Oncolytic Poxviruses. Ann Rev Virol. 2014;1(1).
- Heldin C-H, et al. High interstitial fluid pressure — An obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806-813.
- Heidbuechel et al. Oncolytic viruses encoding bispecific T cell engagers: A blueprint for emerging immunovirotherapies. J Hematol & Oncol. 2021;14(1):63.
- Souza C, et al. Prodrugs for targeted cancer therapy. Expert Rev Anticancer Ther. 2019;19(6):483-502.
- Nguyen KG, et al. Localized interleukin-12 for cancer immunotherapy. Front Immunol. 2020;11.
- Luo Y, et al. Tumor-targeting oncolytic virus elicits potent immunotherapeutic vaccine responses to tumor antigens. OncoImmunology. 2020;9(1):1726168.
- Wennier ST, et al. Bugs and drugs: Oncolytic virotherapy in combination with chemotherapy. Curr Pharm Biotechnol. 2012;13(9):1817-1833.


