ABOVE: By tweaking existing fluorophores, Luke Lavis and his colleagues at the Howard Hughes Medical Institute’s Janelia Research Campus produced dyes that were brighter and longer lasting. Then, they gave them away to other researchers for free. JONATHAN GRIMM
Jay Bradner had been a physician for several years, specializing in the treatment of advanced cancer patients at the Dana-Farber Cancer Institute in Massachusetts, when his own father was diagnosed with pancreatic cancer in 2007. At the time, “there wasn’t a drug I could prescribe for cancers with this underlying etiology,” Bradner says. This was in part because the particular form of cancer his father had was caused by mutations in genes that were considered “undruggable” with existing treatments, he explains.
Bradner had already started retraining as an organic chemist and chemical biologist before his father died only months after his diagnosis, but he tells The Scientist in an email that his family’s struggle with cancer “galvanized the decision” to take on more-translational research. He joined the Center for the Development of Therapeutics at the Broad Institute of MIT and Harvard and began working with bromodomain inhibitors, then an emerging class of small molecules that target proteins involved in many cancers.
The compound he helped develop, named JQ1, proved effective at inhibiting one such protein, BRD4, in early experiments. Exposing mice to JQ1 dampened the animals’ inflammatory response to cancer and reverted many of their tumor cells back to a healthy phenotype—in essence, JQ1 made those cells forget they were cancer. Bradner says that he sensed the molecule’s utility in basic research and, in consultation with his colleagues, decided to do something unusual: rather than patenting the molecule or selling it to a commercial company for profit, they decided to release it freely. They sent samples to colleagues who asked for it and published details on JQ1’s structure and synthesis in a paper that has since racked up thousands of citations.
Pretty soon, “a bunch of people started publishing findings” based on work with the compound, says Ross Levine, a physician-scientist at the Memorial Sloan Kettering Cancer Center and a collaborator of Bradner’s who received an early iteration of JQ1. Those findings came from research areas as diverse as neurodegenerative disease, fibrosis, stem cell biology, and spermatogenesis. “There was way more science than his lab could have ever done,” Levine tells The Scientist. In the cancer therapeutics field, one JQ1 derivative was used to develop a myelofibrosis drug that is currently being tested in a Phase 3 clinical trial by Massachusetts-based Constellation Pharmaceuticals, recently acquired by the German biotech MorphoSys. (Levine previously consulted for MorphoSys on projects unrelated to JQ1, and received funding from Constellation for preclinical research in his lab on another BRD4-targeting drug candidate.) “By just making the reagent available, it promoted more people to work on an important problem and advance the field much faster,” Levine says.
While a citation is appreciated, there are no authorship requirements, no collaborations expected . . . and no fees.
Bradner’s choice to make JQ1 available for free runs counter to the way science is typically done, where experimentalists purchase their reagents from commercial entities and closely guard the intellectual property that results from their research. Sharing reagents has its perks, including direct interactions between scientists that can foster new collaborations, and cost savings that allow researchers to take more risks with their experiments. While freely sharing reagents is not without its pitfalls for the researchers who orchestrate these projects, those who spoke to The Scientist say that the difficulties are trumped by the contributions they make to their own fields—as well as to the fields of their collaborators—by sharing more openly. The decision to make JQ1 so widely available, according to Bradner, now the president of the Novartis Institutes for BioMedical Research, “is the gift that keeps on giving, and we absolutely should be doing this more frequently.”
Beyond commercial suppliers
Alexandre Bisson was a postdoctoral researcher at Harvard University when, in 2014, he started running into serious difficulties. As a cell biologist interested in how microorganisms grow and divide, he relied on microscopy to visualize individual cells and molecules in hay bacillus (Bacillus subtilis). In particular, he was looking for a ring of polymers that determines the site of pinching during division. Bisson was using fluorescent proteins to track individual molecules, but because the bacteria divide so rapidly, “every 20 minutes, they turn over all their components,” he says, and only a subset would fluoresce properly. “Even if I was very patient . . . I’d have to do hundreds of experiments to collect a decent number of data points to infer things.”
After six months of struggling, during which he tried 12 different fluorescent proteins and engineered more than 30 bacterial strains, Bisson wasn’t certain he’d be able to complete his project. The work had the capacity to completely change how scientists study cell division in bacteria, he says, and yet “I felt I was failing.”
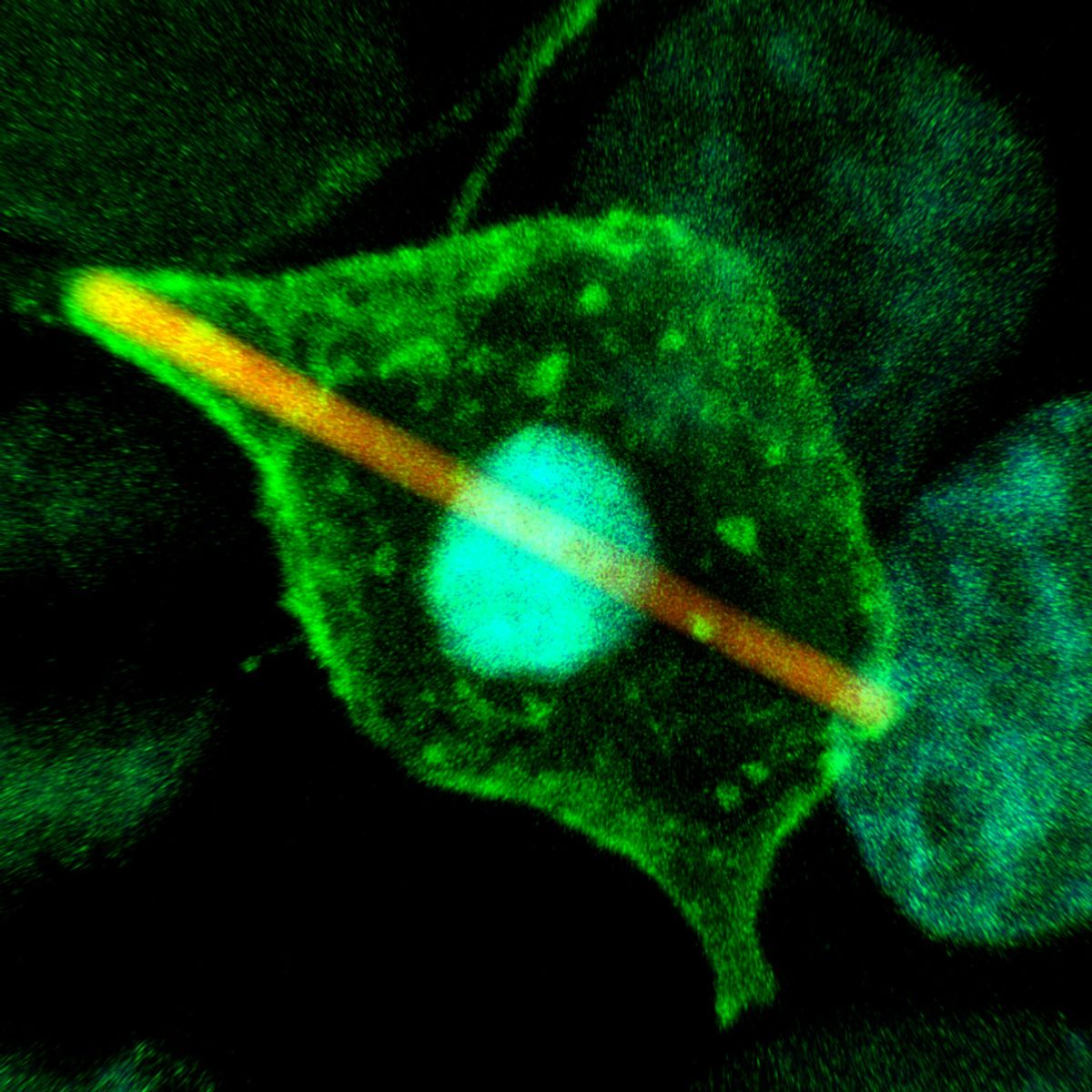
Fortunately, help was around the corner. His adviser had recently attended a talk by Luke Lavis, an organic chemist at the Howard Hughes Medical Institute’s Janelia Research Campus. By making a structural modification to an existing fluorophore, Lavis’s team had recently produced a set of dyes that were brighter and longer lasting. There was an obvious commercial application, Lavis tells The Scientist, but they only briefly considered selling. “We can make these things and because we invest in the chemistry, we can make them cheaply, so we decided, ‘Screw this, let’s just give everything away.’”
Lavis made his dyes available by request, developing a portal that tracks the lab’s inventory. The group keeps a freezer stocked with dyes and often saves intermediate products in case they might be useful. Lavis asks for nothing in return—while a citation is appreciated, there are no authorship requirements, no collaborations expected in exchange for product, and no fees. Since starting in 2017, Lavis estimates that he has sent roughly 13,000 aliquots to more than 500 labs in 33 different countries.
In an eLife article that Lavis wrote about his experience, which he describes as an example of “open science,” he recalls getting an email from Bisson that read, “These dyes are so bright, I’m crying at the microscope.” Speaking to The Scientist, Bisson, now a cell biologist at Brandeis University in Massachusetts, credits Lavis’s reagents with saving his project and jumpstarting his career. “The first time I tried this experiment [with the dyes], it took a week to get the results. Six months of struggling, shortened to a week by the proper reagent,” he says. “That experiment was very important for my paper to get published in Science and for me to get the job that I have now.”
Countless hours go into successfully designing, synthesizing, optimizing,
and shipping new reagents.
Another researcher who works with Lavis’s dyes, Harvard University chemical biologist Adam Cohen, notes that establishing a relationship with Lavis let his lab pursue new research that might otherwise have been out of reach. His team recently posted a preprint detailing a new method for tracking cell activity. Similar to how tree rings reveal a tree’s age and the conditions in which it grew, Cohen’s new technique produces a “stick” with differently-colored stripes, each of which represents the duration of a pulse of molecular activity.
“This was very much a digression from my lab’s prior activities . . . but now it’s become a major new direction for my lab and I’m hoping to devote much of our effort to this in the coming years,” Cohen tells The Scientist in an email, adding that the dyes were “absolutely essential” to establishing this line of research. “At first I was very hesitant about doing these experiments because I didn’t want to use too much of these precious dyes and the commercially available ones were so expensive. Luke was a key brainstorming partner here—his encyclopedic knowledge of the dyes and his willingness to share ideas as well as reagents were critical to the success of this effort.”
Some recipients of scientist-made reagents cite this personal connection with the reagent designer as one of the biggest pluses of working with other researchers instead of commercial suppliers. For a start, scientists who are representing their own products may have an added impetus to ensure that they function as expected and produce consistent results. “A company reports whatever data it wants, and it’s up to the community to be equipped and informed enough to [interpret their findings],” says John Janetzko, a biochemist and postdoctoral researcher at Stanford Medicine. And although commercial companies follow rigorous quality control measures, there can be “a huge advantage when you get [a reagent] directly from someone who’s invested in the science. If you get a dye from Luke Lavis, he’s not interested in giving you something that he scraped off the floor, because it has a train of accountability to him.”

Working directly with a chemist means that biologists who may not fully understand the chemistry underlying specific reagents can also tap the developers themselves to troubleshoot experiments, interpret outcomes, or even develop niche reagents from scratch. Janetzko notes that this better comprehension of the biochemistry behind their experiments can help biologists design better research, too. As a chemist, “you know how [a reagent] should behave, you know the details of it, and so I think you bring more to the table,” he says.
These initial interactions sometimes spark long-term collaborations as well: Bisson, for example, still collects data using Lavis’s probes years later, and says that given their long-standing relationship, he’s willing to demo new products from Lavis. “Luke might reach out and ask if I want to try something that he acknowledges may not work, but I’m happy to try it.”
Acknowledging a challenge
Despite the many feel-good stories stemming from reagent sharing, agreeing to make things publicly available is not without its complications. Countless hours go into successfully designing, synthesizing, optimizing, and shipping new reagents. Asked what he might do differently in hindsight, Lavis tells The Scientist that he wishes he’d implemented a better organizational system more quickly. “For the first few years, it . . . was maybe not the best use of my time, but it was really fun,” he says, recalling how he used to spend weekends packaging vials. “Eventually you reach some threshold where you have to improve the efficiency.”
Hermen Overkleeft, an organic chemist and chemical biologist at Leiden University in the Netherlands, also grappled with the logistics of sharing materials. Overkleeft came up through academia as a chemist working in biology labs, and he enjoyed helping his colleagues design experiments. Today, he still relies on these types of collaborations to drive his research on the role of glycosidases and proteases in disease. “I do the basic biochemical experiments myself, and then reach out to collaborators to look at very specific physiological questions,” he tells The Scientist.
Scientists who are representing their own products may have an added impetus to ensure that they function as expected and produce consistent results.
But as his lab has grown, the internal work needed to manage his collection of available probes has grown too. Where he once had roughly 10 to 20 products advertised by word of mouth, he now has thousands, and each order can take an hour to process. Overkleeft’s lab doesn’t see quite the traffic that Lavis’s does, but it still counts dozens of orders a year. And because many of his compounds “tend to be relatively unstable,” he says, the team has to store them dry and rehydrate them when requested.
Of course, releasing products in this way also means that labs miss out on money, and almost no one is able to make everything free. Researchers who spoke to The Scientist made it clear that they do still partner with commercial entities or have even gone on to start their own companies to commercialize some of their output. Oftentimes, if the reagent is requested frequently enough, it just makes sense to outsource it. Licensing some products for commercial use also helps recoup some of the overall costs of processing orders. Matthew Bogyo, a chemical biologist at Stanford Medicine, tells The Scientist in an email that “we have sold companies a supply . . . for a set price so that they can package and sell it for a profit,” adding that “this is simply because the major cost to my lab to make the reagent available is the effort of preparing a sample, packaging it and sending it out.”
Several researchers who spoke to The Scientist also point out that academia has been slow to embrace this more altruistic sharing of materials. Sloan Kettering’s Levine notes a “paucity” of successful examples like JQ1, while Bisson calls these instances “something really rare and special.” He adds that it’s likely that some sort of cultural change will be needed before the practice becomes mainstream. Among chemists especially, where a reagent is often the end product of years of research, prompting other scientists to share their work without tacking on qualifiers around coauthorship remains a challenge. While researchers often claim to be amenable to requests for reagents, it doesn’t always play out in practice.
Nevertheless, scientists who benefit as recipients of shared materials may gain a newfound appreciation for this way of working, and some go on to make changes in their own practices.
When Bisson started his lab, for example, he immediately began building open practices into his workflow. While he doesn’t synthesize reagents himself, Bisson says that he is updating his website to make his strains lists, methods, and protocols—even those he has yet to publish—freely available. “Because of people like Luke and others, I learned that it feels good to share,” Bisson tells The Scientist. “I know how I felt when I was screaming into the microscope, when after six months of struggling, I put a little dye inside those cells and it was so bright that I didn’t have to do a lot to get the results that I was seeking. I only got that because of open science.”
Membership Open House!
Enjoy OPEN access to Premium Content for a limited timeInterested in exclusive access to more premium content?




