ABOVE: The Scientist Staff
With the COVID-19 pandemic dragging toward a most unwelcome third year, it’s not surprising that the biomedical community has continued to focus on diagnosing and treating the disease. The list of this year’s Top 10 Innovations winners reflects these shared goals with a couple of products that can help researchers better understand the biological realities of SARS-CoV-2 infections, interrogating cells neighboring those infected with the virus, for example, and the immune system’s reaction to it over time.
But 2021’s innovation landscape also includes laboratory and clinical products that provide a more expansive view on biology. The winners of this year’s competition include an implantable miniscope that can track activity in the brains of freely moving organisms; a microfluidic device that aims to recapitulate whole-organism physiology; and a few products that build on the emerging trend toward characterizing individual cells, with the added components of spatial information or multi-omics.
Since the last installment of our Top 10 innovations, the world has witnessed the successful deployment of multiple COVID-19 vaccines, and those are, in their own right, truly awe-inspiring innovations. In a way, it’s heartening that scientific advances have continued to occur in spaces outside of the crucial coronavirus focus. It suggests that the global biomedical apparatus is robust enough to address a pressing and pointed concern while not losing ground in fields not directly related to that crisis.
Here are the breakthroughs and advances that, thanks to the careful consideration of our panel of independent judges, have won a spot in our annual Top 10 Innovations competition.
InscopixnVue™ System


Weighing in at two grams, the nVueTM System is about the size of a Lego brick. This “miniaturized microscope” relies on red and green fluorescent indicators targeted to neurons to trace calcium ion influx and, in turn, the activity of two different neuronal populations in freely moving animals, according to Alice Stamatakis, director of applications at Inscopix, the company that makes the nVue. Thus far, researchers have mounted nVue on the heads of rodents, birds, and monkeys.
The miniscope offers another advantage, Stamatakis adds: longitudinal deep-brain imaging, wherein the same cells can be analyzed over multiple imaging sessions. Two-photon microscopy also allows simultaneous imaging of two neuronal populations, but it is mostly limited to the brain cortex and requires animals to be constrained by the head, compromising the study of behavior, she says. “[nVue] is going to give neuroscientists an unprecedented view into how these different brain signals communicate and talk with each other during naturalistic behaviors.” The system’s built-in data acquisition and processing software helps complete the picture.
Beyond basic biology, the dual miniscope can aid translational research for neuropsychiatric and neurodegenerative conditions, such as anxiety or Alzheimer’s disease. Kelly Tan, a neurologist at the University of Basel, Switzerland, uses the nVue system to study communication between neuronal populations in a mouse model of Parkinson’s disease. “It’s been a breakthrough for circuit neuroscience,” Tan, who highlighted the dual miniscope’s merit in a video and webinar for Inscopix, tells The Scientist.
In addition to tracking two neuronal populations, researchers can use the miniscope to juxtapose fluorescence signals from calcium influx in neurons and plasma in blood. This allows for analysis of the relationship between neuronal activity and vascular dynamics, including capillary diameter and red blood cell velocity, in the brain. Stamatakis says that Inscopix is now working to layer electrophysiology recordings and enhanced behavioral analyses into the miniscope.
Inscopix declined to provide a price for the system, explaining that the cost varies regionally.
WILEY:“The innovation is in what it allows researchers to do, which is to follow two activities in the brains of freely behaving animals over time.”
CN BioPhysioMimix™ OOC Multi-Organ Microphysiological System


CN Bio released the PhysioMimix™ OOC Multi-Organ Microphysiological System in March 2021 after about 10 years of research and development through a collaboration between the Defense Advanced Research Projects Agency (DARPA) and MIT. A microfluidic organ-on-a-chip platform undergirds the PhysioMimix™ Multi-Organ System and allows scientists to connect individual organ-on-a-chip models—for example, a liver model with a gut or lung model also developed by CN Bio—for disease research and drug development, explains company CEO David Hughes.
Each chip contains millions of organ-specific human cells that can be connected in a multi-well plate format. The system mimics biological conditions by allowing media recirculation to different organ culture compartments, says Hughes; this “creates data that’s more predictive of human response” compared to insights gleaned from animal models. He adds the product is geared toward “providing more-accurate, human-relevant information to researchers in the pharmaceutical industry.”
Martin Trapecar, a Johns Hopkins immunologist and bioengineer, uses this system in his lab to study the effects of autoimmune and autoinflammatory diseases on gut and liver tissue. He says that the product presents a more realistic model to develop regenerative and personalized therapies and “eliminates a lot of the problems with studying immunology. . . . The other benefit is it gives me very granular insight into how tissue-tissue and tissue-immune interactions inform the behavior of the whole system.” According to a company announcement, CN Bio considers this technology a milestone toward an eventual “body-on-a-chip” system.
The company declined to share the price of the system.
HOCKBERGER: “[T]his product improves on the original system (launched in 2018) to enable a wider user base. . . . Cool!”
Vizgen MERSCOPETM


One of two spatial genomics tools in this year’s Top 10, Vizgen’s MERSCOPETM is the only single-cell spatial genomics instrument currently available for purchase. Designed to conduct and analyze multiplex error resistant fluorescence in situ hybridization (MERFISH) experiments, the platform detects RNA transcripts from hundreds of genes across intact tissue and returns imaging and expression data at subcellular resolution.
The product was developed as “a new sort of research tool that gives people this unprecedented view into biological systems,” says Vizgen cofounder and director of technology and partnerships George Emanuel. “You know exactly where each transcript is with 100-nanometer accuracy.”
The Salk Institute’s Pallav Kosuri, who is using MERSCOPETM for detailed cardiac tissue imaging, says it’s useful to work directly with the instrument, adding that while sample prep is laborious, the analysis is fully automated by MERSCOPETM. “Everything has worked really smoothly,” says Kosuri, who did his postdoc in the Harvard University lab where the technology was developed but was not involved in the work. When he’s needed technical support, “the company has been really good at dedicating time and effort to troubleshoot with us.”
One $300,000 purchase includes the automated instrumentation, plus data visualization software and other infrastructure needed to run MERFISH experiments; reagents and probes for researchers' genes of interest cost extra. The first units were shipped in August of this year.
Kosuri says Vizgen can price the platform so high because currently, they “are the only ones doing this.” But it’s prohibitively expensive for many labs. “As an investigator, it’s super steep.”
KAMDAR: “MERSCOPE is the first commercially available high-plex, single-cell spatial genomics platform for spatially profiling gene expression across whole tissues and resolving individual transcripts with nanometer-scale resolution. The coordination of gene expression and spatial profiling opens new windows in the precise architecture of a cell.”
Emulate Brain-Chip
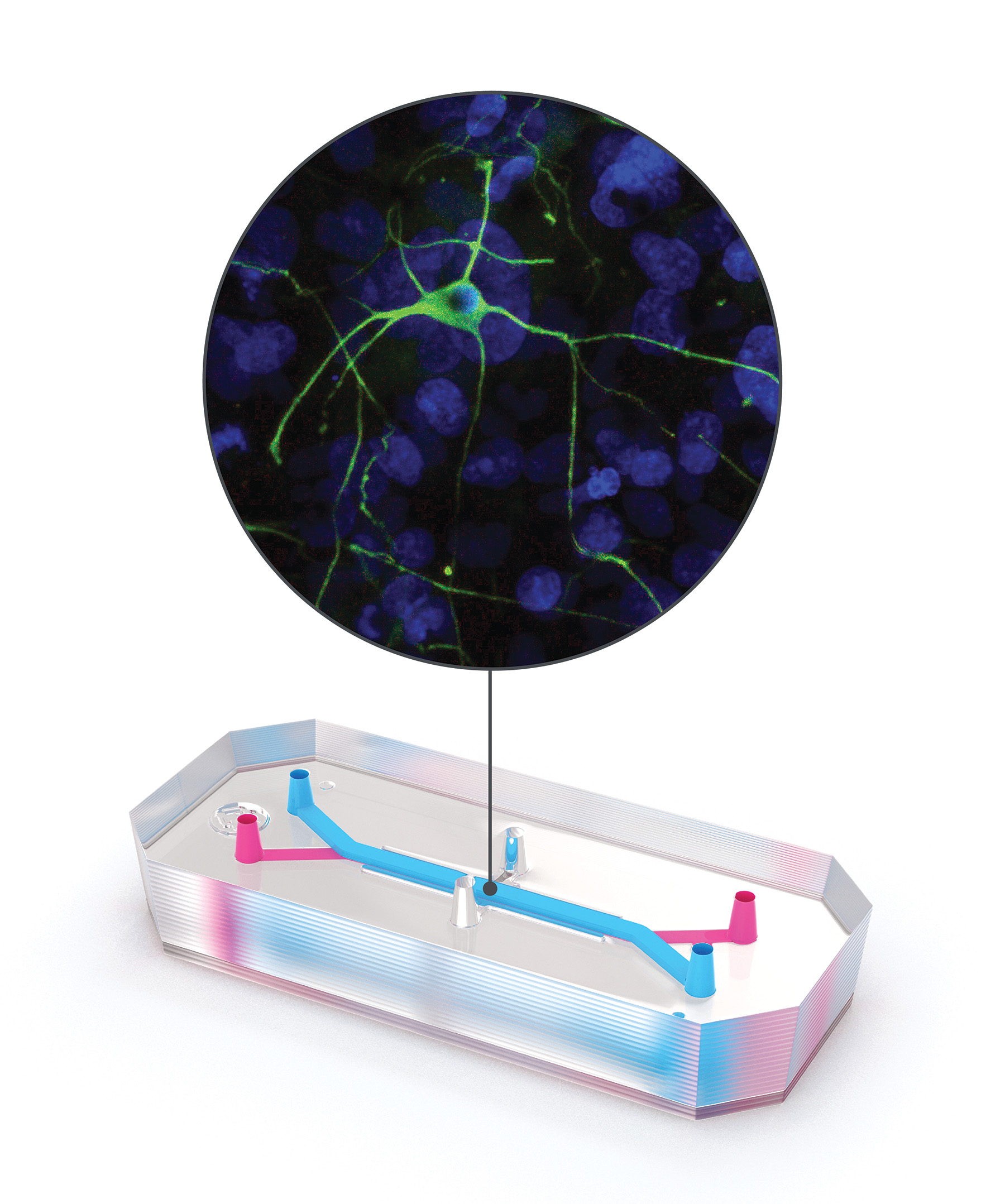

The blood-brain barrier (BBB) poses a challenge for the development of drugs that target the brain. Layers of cells that line the blood vessels of the brain evolved to help keep out toxins or other molecules that could potentially harm this vital organ, but they also block the passage of most therapeutics. With a decade-long history of developing organ-on-a-chip models, biotechnology company Emulate set out to create one that could accurately model this barrier and the structures on either side of it.
“This is our most complex and most adventurous chip because it not only has the endothelial cells, it has astrocytes, pericytes, microglia, and neurons,” says Lorna Ewart, the company’s executive vice president of science.
The Brain-Chip, which was released in December 2020, consists of two channels embedded in flexible rubber polymer. One channel is lined with stem cell–derived endothelial cells, representing blood vessel walls, and the other is lined with neurons and glia. Midway along the chip, the two channels come into contact. As fluid moves through the “blood vessel” channel, scientists can study how molecules interact with and move to the other channel—effectively crossing the BBB—and how they affect structures there.
The Brain-Chip can model both healthy and unhealthy neurological states. At Cedars Sinai in Los Angeles, developmental biologist Michael Workman and colleagues have been using patient-derived stem cell lines to create models of neurodegenerative diseases such as Parkinson’s on the Brain-Chip. “Each one of these chips is like a little patient avatar,” he says. “We do have a large interest in that personalized health and precision medicine approach, and see these microfluidic chips as a way to push more towards that.”
Emulate declined to provide a price for Brain-Chip, as it depends on end users’ requirements.
WILEY: “Very sophisticated organ-on-a-chip for brain research, providing a new and powerful approach for investigating mechanisms of neuro-inflammation and blood-brain barrier function.”
Q BioGemini and Mark I


The Gemini platform and Mark I scanner by Q Bio were introduced in April 2021 as a way to monitor patient health more comprehensively than has previously been possible in healthcare. Although it is not yet widely available, the company is rolling it out with a limited number of patients and doctors as part of a pilot program.
The Mark I prototype scans the entire body with the patient sitting, standing, or lying down, using magnetic resonance imaging (MRI), which creates high quality images without radiation—unlike X-rays, computer tomography (CT), or positron emission tomography (PET). A scan with Mark I takes only about 15 minutes, compared with traditional full-body scans that can take more than an hour. The imaging information is uploaded to the Gemini platform, along with medical records, genetics data, and traditionally acquired tests of blood, urine, saliva, and vital signs. Putting all these data together, the platform creates a “digital twin” of the patient’s anatomical structures, vital signs, and body chemistry.
By cataloging these data, small changes can be compared over time, and mathematical models could predict problems before they occur, says Jeffrey Kaditz, founder and CEO of Q Bio. This could allow doctors to efficiently triage patients’ needs based on annual scans. Currently, an annual patient membership costs $3,495 and includes a scan and consultation. Q Bio has not yet applied for FDA approval, and the company does not accept health insurance.
“Our aim is to bring a sea change in how health care is delivered on a large scale,” William Stanford, chief medical and scientific officer for the Beverly Hills Institute for Precision Medicine, says via email, adding that Gemini is the “perfect adjunct” to the facility’s multi-omic data collection efforts. The approach can be a bit cumbersome, he notes, as his patients must fly from Los Angeles to northern California, then drive to Q Bio’s facility in Redwood City for the scan—all of which takes around eight hours, round trip. The results come back two weeks later and can be sent to the patient’s primary doctor.
WILEY: “This is a software-hardware platform to create a digital representation of a patient that can be stored and analyzed over time. The very fast (<15 min) whole-body scanner is key. This is truly a groundbreaking innovation in developing a digital framework for understanding human physiology and aging.”
10xGenomics Chromium X


Chromium X is 10x Genomics’s newest instrument for single-cell analysis. Users load cells in suspension and add reagents and a partitioning oil into the microfluidic chip, which goes into the benchtop instrument. The resulting droplets, or Gel Bead-In-Emulsions (GEMs), each contain a single cell, a single barcoded Gel Bead, and a reagent, and are ready to be sequenced and used in assays offered by 10x Genomics, including gene expression analysis, epigenetic profiling, and immune cell profiling. Each GEM carries a unique barcode, allowing the user to later link results back to a single cell.
The Chromium X is the latest in a long line of products from 10x Genomics that have won top spots as Top 10 Innovations. In 2019, the firm’s single-cell, droplet-based sequencing system made the Top 10, and in 2020, the Chromium Single Cell Multiome ATAC + Gene Expression assay won a spot. Chromium X improves on previous products because of its flexibility, says Jens Durruthy Durruthy, associate director of product management–single cell at the company. “Chromium X is scalable and can be used both for low-throughput assays, with hundreds of cells, as well as for high-throughput assays with up to one million cells.”
Sisi Chen, director of the Single-Cell Profiling and Engineering Center at Caltech, notes in an email to The Scientist that the high-throughput capability of the Chromium X is essential to her research. She uses the Chromium X, which was launched in July 2021, to explore how therapeutic compounds influence the human immune system, and the system allows her to simultaneously stimulate 1 million immune cells, each with one of up to 100 different therapeutics, and track their responses. “We want to profile the [immune] system across hundreds to thousands of different unique conditions,” says Chen.
In the US, Chromium X is available from $100,000. With high-throughput assays, users can get the cost per run down to 2 cents per cell.
HOCKBERGER: “10X Genomics is back with a new high-scale, high-resolution version of its flagship instrument, Chromium. The latest product democratizes access to high throughput, single-cell analysis of gene expression and immune profiling by offering it at an affordable price. Well done!”
The Native Antigen Company SARS-CoV-2 Neutralization Assay Development Kits


A few years ago, the World Health Organization added “Disease X” to its short list of emerging diseases—a placeholder for unknown pathogens with pandemic potential. Researchers at The Native Antigen Company, a UK-based group that designs reagents for infectious disease research, speculated in November 2019 that one candidate might be a coronavirus, one that would likely arise in Asia and spread from animals. Then came SARS-CoV-2.
By spring 2021, The Native Antigen Company had developed a coronavirus neutralization assay to determine a serum sample’s level of antibodies that bind, and therefore neutralize, the virus. The assay uses synthetic versions of the SARS-CoV-2 spike protein’s receptor binding domain and its target, the mammalian ACE-2 receptor, and pairs them with an ELISA-based platform that quantifies the neutralizing ability of the antibodies via a color change, explains the company’s Commercial Director, Andrew Lane. Researchers can use this tool to probe how patients respond to infection and to study vaccine efficacy, among other applications, he adds.
The kit doesn’t require live virus and is speedy compared to methods that use benign, engineered viruses called pseudoviruses, according to Lane. Since the first kit was released in April 2021, the company has produced assays for five variants. “It’s a bit like a plug-and-play system for us. We can make kits with different variants quite quickly,” Lane says. Each kit analyzes 960 samples and costs $2,728.
“Overall, we’re very happy with its response,” says Matthew Edmans, a postdoctoral researcher at the University of Oxford who is using the assay to study how patients on immunomodulatory drugs respond to SARS-CoV-2 infection. Edmans also uses pseudoviruses, but agrees they can be “quite complicated,” while The Native Antigen Company’s assay “is just a lot faster and more straightforward to run.”
KAMDAR: “Needed to assess the protection and longevity of patient immunity to emerging variants. This is all done without the need for BSL3 facilities, thereby providing a safer alternative for these critical public health questions.”
Mission Bio Tapestri Single-cell Multi-omics Solution


Mission Bio’s Tapestri Single-cell Multi-omics Solution, launched in October 2020, is a process for single-cell analysis that allows users to consider DNA sequence and proteomic information simultaneously—an innovation on traditional setups that required separate systems to analyze nucleic acids and proteins, which could therefore not be correlated at the single-cell level. Mission Bio’s Tapestri Precision Genomics Platform earned a spot in The Scientist’s Top 10 Innovations of 2018 and was the first high-throughput instrument for single-cell DNA sequencing sample prep. “We were then able to add other analytes, such as proteins, subsequently,” says Adam Sciambi, Mission Bio’s cofounder and senior director of technology & systems.
With the Tapestri Single-cell Multi-omics Solution, assays for DNA and protein are combined in a single integrated workflow that can analyze up to 10,000 cells at a time. The Tapestri instrument uses microfluidics to capture individual cells in droplets that contain both the reagents for DNA sequencing and antibodies for tagging cell-surface proteins, plus a barcoding bead. “The result of our platform is every piece of DNA comes out labeled as telling you which droplet it came from,” says Sciambi. The DNA is then sequenced using next-generation sequencing, and the cells’ surface proteins are characterized. Mission Bio declined to disclose the cost of the platform.
Molecular biologist Jan Cools of the VIB-KU Leuven Center for Cancer Biology in Belgium has used the Tapestri platform to investigate mutations underlying acute lymphoblastic leukemia (ALL). He is now planning to use Mission Bio’s Tapestri Single-cell Multi-omics Solution to obtain additional information on cell-surface markers, a setup he says will be especially useful for studying a different type of blood cancer, acute myeloid leukemia (AML). In AML, some leukemia cells are known to be more stem cell–like, while others are more differentiated, a difference that could be captured by looking at cell surface markers and sequencing data, Cools says.
KAMDAR: “Tapestri is the only commercialized multiomics platform capable of analyzing DNA and protein simultaneously from the same sample at single-cell resolution. The real power is the ability to generate correlation data between the genome, transcriptome and the proteome.”
Cardea Bio CRISPR-SNP-Chip


Cardea Bio’s CRISPR-SNP-Chip is the first device capable of detecting single base differences in DNA without generating millions of copies of the DNA first. “We can do DNA tests without the need of a DNA lab,” explains Cardea CEO Michael Heltzen.
The latest of Cardea’s biological processing units, or BPUs (analogous to the CPUs that underlie computer technologies), the chip is an updated version of the company’s CRISPR-Chip™, which already allowed for rapid, amplification-free detection of large, disease-associated sequence variants and transgene insertion success, among other applications, says Keck Graduate Institute biomedical engineer and Cardea Chief Scientific Officer Kiana Aran. She explains that both versions are composed of a CRISPR-Cas system tethered to a graphene transistor. When the Cas enzyme’s guide RNA binds to the correct sequence, it pulls the DNA closer to the transistor. Because DNA is negatively charged, this generates an electronic signal in the semiconductive graphene that can be digitally read. “You let the biology do what it’s good at, and then you sense it with our sensor,” says Aran. “We use the power of biology as technology.”
Giving the chip the ability to detect single nucleotide polymorphisms (SNPs) involved replacing the Cas enzyme with a more sensitive version and upgrading the data analysis, Aran notes. In an April paper, the team demonstrated the updated chip’s ability to detect SNPs that underlie sickle cell anemia and amyotrophic lateral sclerosis (ALS), though the potential applications are bounded only by creativity, the authors write. Its most immediate use is for quality control of gene editing for medicinal or agricultural purposes, Aran says.
Those interested in using the chips can apply for Cardea’s partnership program. While the exact cost depends on the application, Heltzen notes that the price per chip has dropped to tens of dollars from the thousands they were a few years ago.
HOCKBERGER: “Another game changer for clinical diagnosis.”
Resolve Biosciences Molecular CartographyTM Single-Cell Spatial Analysis Service


Spatial biology addresses how cells function in the context of tissues. While single-cell sequencing methods have permitted many advances in this field, they lack the resolution to provide 3D data at subcellular scales, and involve destroying the tissue sample. Resolve Biosciences’s Molecular Cartography™ Single-Cell Spatial Analysis Service, the second spatial genomics tool in this year’s Top 10, instead offers fluorescence in situ hybridization (FISH) to create high-resolution images of what genes are expressed—down to the subcellular level.
The mail-in service, launched June 2 in North America and Europe, detects individual RNA transcripts inside intact tissues. “We can interrogate pretty much any tissue you can put on the slide,” says company CEO and cofounder Jason Gammack, adding that the platform can analyze 24 samples simultaneously. Costs depend on project specifications, but most customers can generate sample data for around 4,000 euros, he says. That includes a meeting with a customer technology adviser to define project scope and a sample prep kit in a return-mail box. Researchers receive a summary report and data on their chosen genes—the platform lets researchers visualize up to 100—in about four weeks; the adviser also helps researchers interpret the data.
Jean-Christophe Marine of the VIB-KU Leuven Center for Cancer Biology has used the service through an initiative at his institution that supports early access to new technologies. “We are very satisfied by the data,” says Marine, who studies intratumor heterogeneity in melanoma. “[The] vast majority of the probes worked, and . . . you have a nice resolution.” The service is well-priced, he adds, although his team has only been able to analyze mouse samples due to restrictions on mailing human samples.
In the future, Resolve Biosciences plans to make the whole Molecular CartographyTM platform available for researchers to operate themselves. The company will expand what types of molecules can be imaged, too, Gammack says, with proteins up next. “We’re actively developing that chemistry right now.”
KAMDAR: “The technology has potential to help researchers better understand human brain development, cell type evolution, and how the SARS-CoV-2 infection affects neighboring cells over time.”
THE JUDGES

Philip Hockberger
Associate professor of neuroscience in the Feinberg School of Medicine at Northwestern University. He is recognized internationally for his leadership role in research core facilities and for promoting the careers of core scientists.

Kim Kamdar
Managing partner at Domain Associates, a health care–focused venture fund that creates and invests in biopharma, device, and diagnostics companies. She began her career as a scientist and pursued drug discovery research at Novartis for nine years.

H. Steven Wiley
Senior research scientist and laboratory fellow at Pacific Northwest National Laboratory. He published some of the earliest computer models of receptor regulation and is known for developing a variety of quantitative biochemical and optical assays as a basis for validating computational models of cell processes.
Editor’s Note: The judges considered dozens of entries submitted for a variety of life science products by companies and users. The judging panel evaluated submissions with only basic instructions from The Scientist, and its members were invited to participate based on their familiarity with life science tools and technologies. They have no financial ties to the products or companies involved in the competition. In this issue of The Scientist, any advertisements placed by winners named in this article were purchased after our independent judges selected the winning products and had no bearing on the outcome of the competition.
Correction (December 1): The original version of this article stated that DNA is positively charged, when in fact it is negatively charged. The Scientist regrets the error.
Membership Open House!
Enjoy OPEN access to Premium Content for a limited timeInterested in exclusive access to more premium content?







